

The cookie is used to store the user consent for the cookies in the category "Other. This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary".
The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category. In addition to certain standard Google cookies, reCAPTCHA sets a necessary cookie (_GRECAPTCHA) when executed for the purpose of providing its risk analysis. These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. Lightning Lightning is a phenomenon that consists of the discharge on the ground of static electricity that has been generated in the clouds by the friction of the ice crystals they contain.
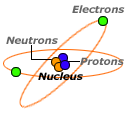
The water in the air helps the electrons to quickly move off your body because water is a conductor that’s why we can’t build up enough static charge. During the summer, the air is more humid. Winter-Summer We usually only notice static electricity in winter when the air is very dry. This is called the principle of conservation of charge. Electrons just move from one place to another. You can see for yourself by experimenting with them.Ĭharge is neither created nor destroyed … When we charge an object with static electricity, we neither create nor destroy electrons, nor do we make protons appear or disappear. Under ideal conditions, if two of these materials rub against each other, the one higher on the list gives up the electrons and becomes positively charged. This classification is called the triboelectric series. What material will release electrons and what will it accept? Scientists have classified materials based on their ability to hold or give up electrons. When we rub two materials together, electrons from one material move to the other. Scientists believe that it is not friction that causes the electrons to move, but simply the contact between the two materials friction only increases the contact area between them. The more you rub two objects together, the more electrons move, and therefore the larger the charge that builds up. Touch a doorknob and… ZAP!! The electrons move from you to the doorknob and you get a static shock because your nerve receptors are stimulated. When you walk across a rug, especially synthetic ones, you’re rubbing against it and its electrons move from the rug to you. As they are tightly rooted in our heads (luckily), the best thing they can do is to stand up, away from all the other hairs. Therefore, the hairs try to move away from each other. Things with the same charge repel each other. When you do that, the hat rubs against your hair, and some electrons move from your hair to the wool and in this way each of the hairs become positively charged. This explains why your hair stands on end when you take off a sweater or a wool hat. If two things have opposite charges, they attract each other if they have like charges, they repel each other. Static electricity is the imbalance of positive and negative charges. When charges are separated like this, it is called static electricity. Atoms that gain new electrons acquire a negative charge, whereas those that lose them become positively charged. But if we rub two objects against each other, some electrons can move from one atom to the other. Protons have a positive charge, electrons have a negative charge, and neutrons have no electrical charge, they are neutral.Ītoms usually have the same number of protons and electrons, so positive charges and negative charges offset each other, so the overall charge of the atom is neutral. One way they are different is the electrical charge. The particles that make up atoms are called protons, neutrons, and electrons, and they are very different from each other. We did not know this when the atom was named.

Although the word atom means “indivisible,” atoms are made up of even smaller particles. All the objects we see are made up of tiny particles called atoms.


 0 kommentar(er)
0 kommentar(er)
